
Gastrointestinal Stromal Tumor Market to Grow Rapidly During the Study Period (2020–2034) Owing to the Launch of Emerging Therapies | DelveInsight
According to DelveInsight’s analysis, the market for GIST is anticipated to increase during the forecast period (2025–2034), owing to the launch of emerging therapies and healthcare spending globally.
/EIN News/ -- New York, USA, April 15, 2025 (GLOBE NEWSWIRE) -- Gastrointestinal Stromal Tumor Market to Grow Rapidly During the Study Period (2020–2034) Owing to the Launch of Emerging Therapies | DelveInsight
According to DelveInsight’s analysis, the market for GIST is anticipated to increase during the forecast period (2025–2034), owing to the launch of emerging therapies and healthcare spending globally.
DelveInsight’s Gastrointestinal Stromal Tumor Market Insights report includes a comprehensive understanding of current treatment practices, emerging gastrointestinal stromal tumor drugs, market share of individual therapies, and current and forecasted gastrointestinal stromal tumor market size from 2020 to 2034, segmented into 7MM [the United States, the EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan].
Key Takeaways from the Gastrointestinal Stromal Tumor Market Report
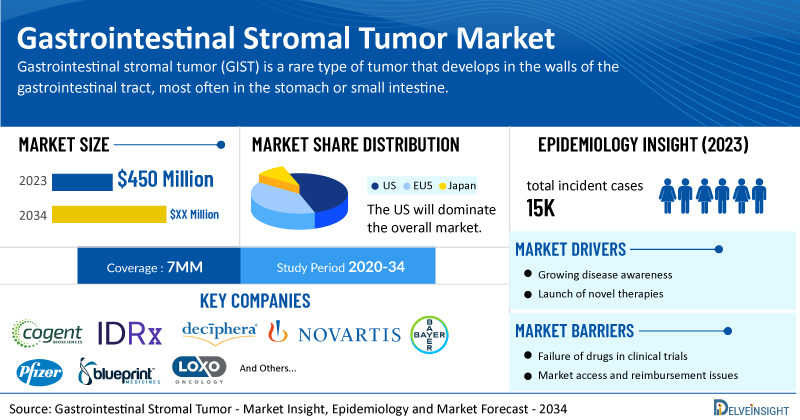
- According to DelveInsight’s analysis, the market size of gastrointestinal stromal tumor in the 7MM was USD 450 million in 2023.
- The United States accounts for the highest GIST market size, approximately 69% of the total market size in 7MM in 2023, in comparison to the other major markets.
- In the assessment conducted by DelveInsight, the estimated total incident cases of GIST in the 7MM were nearly 15K in 2023. The US accounted for the highest number of incident cases of GIST in 2023, and this number is expected to rise sharply due to improvements in diagnostic testing and advancements in genetic testing.
- Prominent companies active in the gastrointestinal stromal tumor treatment space include Cogent Biosciences, IDRx, Deciphera Pharmaceuticals, Novartis, Bayer, Pfizer, Blueprint Medicines Corporation, Loxo Oncology, and others.
- Some of the key gastrointestinal stromal tumor treatments include Bezuclastinib (CGT9486), IDRX-42, Ripretinib, and others.
Discover which therapies are expected to grab the gastrointestinal stromal tumor market share @ Gastrointestinal Stromal Tumor Market Report
Gastrointestinal Stromal Tumor Overview
Gastrointestinal stromal tumor (GIST) is a rare type of tumor that develops in the walls of the gastrointestinal tract, most often in the stomach or small intestine. It originates from interstitial cells of Cajal, which help regulate the GI tract’s motility. GISTs may be either benign or malignant, and treatment typically includes surgical removal and targeted drug therapies.
The development of GIST is primarily driven by mutations in the KIT or PDGFRA genes, though other less common mutations can also play a role. These genetic alterations are typically mutually exclusive. Most GIST cases involve mutations in the KIT gene, while a smaller percentage are linked to PDGFRA mutations. Diagnosing GIST can be challenging due to its genetic variability and diverse presentation. Distinguishing it from other gastrointestinal tumors often requires advanced imaging methods like endoscopy and endoscopic ultrasound. Furthermore, accurate diagnosis relies on detecting key molecular markers such as KIT or PDGFRA mutations through pathological evaluation.
Gastrointestinal Stromal Tumor Epidemiology Segmentation
The gastrointestinal stromal tumor epidemiology section provides insights into the historical and current gastrointestinal stromal tumor patient pool and forecasted trends for the 7MM. It helps recognize the causes of current and forecasted patient trends by exploring numerous studies and views of key opinion leaders.
The gastrointestinal stromal tumor market report proffers epidemiological analysis for the study period 2020–2034 in the 7MM segmented into:
- Total Incident Cases of GIST
- Gender-specific Incident Cases of GIST
- Age-specific Incident Cases of GIST
- Mutation-specific Incident Cases of GIST
- Stage-specific Incident Cases of GIST
- Treatable Cases of GIST by Line of Therapies
Download the report to understand which factors are driving gastrointestinal stromal tumor epidemiology trends @ Gastrointestinal Stromal Tumor Epidemiological Insights
Gastrointestinal Stromal Tumor Treatment Market
The treatment strategy for gastrointestinal stromal tumors follows a structured, tiered approach based on disease progression and the patient’s response to prior therapies. Typically, treatment begins with first-line therapy using imatinib mesylate, a tyrosine kinase inhibitor that targets the most common genetic mutations in GIST—KIT and PDGFRA. While imatinib is effective for many, patients who develop resistance or intolerance may be transitioned to alternatives such as SUTENT (sunitinib). For specific genetic alterations like NTRK fusions, newer therapies such as VITRAKVI (larotrectinib) and ROZLYTREK (entrectinib) are available. In cases involving PDGFRA D842V mutations, AYVAKIT (avapritinib) is a targeted and essential option.
When resistance to imatinib occurs, second-line treatments become necessary. These include sunitinib, STIVARGA (regorafenib), and NTRK inhibitors like larotrectinib and entrectinib. AYVAKIT continues to be pivotal for patients with PDGFRA D842V mutations, offering targeted activity against this resistant subtype.
As the disease advances, third-line options such as sunitinib and regorafenib are employed to manage further progression and resistance. For patients requiring fourth-line and beyond therapies, QINLOCK (ripretinib) is introduced. This switch-control tyrosine kinase inhibitor is designed to tackle a broader range of KIT and PDGFRA mutations, including those linked to multi-drug resistance. Regorafenib also remains a viable treatment in these later stages.
While significant progress has been made in GIST management through targeted therapies, overcoming drug resistance continues to be a major hurdle. The line-based treatment approach allows for individualized care but reflects the ongoing complexity and evolving nature of GIST treatment.
Learn more about the market of gastrointestinal stromal tumor @ Gastrointestinal Stromal Tumor Treatment
Gastrointestinal Stromal Tumor Emerging Drugs and Companies
Companies across the globe are diligently working towards developing novel treatment therapies. Key players involved in the development of promising products, such as Cogent Biosciences (Bezuclastinib), IDRx (IDRX-42), Deciphera Pharmaceuticals (Ripretinib), are developing drugs to treat GIST. Thus, these emerging therapies are expected to further create a positive impact on the market during the forecast period.
Bezuclastinib is a highly selective Type I inhibitor that targets the KIT receptor tyrosine kinase, specifically focusing on oncogenic mutations within the activation loop encoded by exons 17 and 18—including the KIT D816V mutation, which is a primary driver in most systemic mastocytosis cases.
When combined with sunitinib, a Type II inhibitor that targets mutations in the KIT ATP-binding pocket, the duo has demonstrated extended progression-free survival in patients with advanced GIST who have undergone multiple prior treatments.
Bezuclastinib is currently being evaluated in Phase III clinical trials and was granted Orphan Drug Designation (ODD) by the U.S. FDA in 2021. Cogent Biosciences is actively enrolling patients in the PEAK Phase III trial, comparing the combination of bezuclastinib and sunitinib against sunitinib alone in individuals with advanced GIST who have previously been treated with imatinib.
IDRX-42 is an oral tyrosine kinase inhibitor targeting KIT, currently in clinical development for GIST—the most prevalent soft tissue sarcoma of the GI tract. Engineered to inhibit key KIT mutations responsible for tumor progression and resistance, IDRX-42 seeks to address critical gaps in GIST treatment. In the ongoing Phase I/Ib StrateGIST 1 trial, the therapy has demonstrated encouraging anti-tumor effects, including a 53% objective response rate in second-line treatment patients.
A Phase III trial (StrateGIST 3) is planned to further evaluate IDRX-42 in the second-line setting. The U.S. FDA has awarded the drug Fast Track Designation (FTD) and Orphan Drug Designation (ODD) in recognition of its potential in addressing a high unmet medical need.
Ripretinib, a next-generation switch-control kinase inhibitor, is currently approved for use as a fourth-line treatment in GIST and is being studied in Phase III trials for potential second-line use. In the INTRIGUE study, ripretinib was evaluated against sunitinib in patients with advanced GIST following imatinib treatment. While ripretinib showed favorable clinical activity with fewer severe side effects and improved tolerability, it did not outperform sunitinib in progression-free survival.
The ongoing INSIGHT study is focused on patients with KIT exon 11 and 17/18 mutations to determine whether ripretinib can offer greater efficacy than sunitinib in this specific genetic subgroup. These trials underscore ripretinib's promise as a more tolerable and potentially more effective second-line therapy, aiming to fill an important gap in current GIST treatment options.
The anticipated launch of these emerging therapies are poised to transform the gastrointestinal stromal tumor market landscape in the coming years. As these cutting-edge therapies continue to mature and gain regulatory approval, they are expected to reshape the gastrointestinal stromal tumor market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
To know more about gastrointestinal stromal tumor clinical trials, visit @ Gastrointestinal Stromal Tumor Treatment Drugs
Gastrointestinal Stromal Tumor Market Dynamics
The gastrointestinal stromal tumor market dynamics are anticipated to change in the coming years. The GIST market is primarily driven by the rising incidence of gastrointestinal cancers, growing awareness and early diagnosis of rare tumors, and advancements in targeted therapies. The increased adoption of personalized medicine and molecular diagnostics has significantly improved treatment outcomes, especially with the use of tyrosine kinase inhibitors (TKIs) like imatinib, sunitinib, and regorafenib.
Furthermore, many potential therapies are being investigated for the treatment of GIST, and it is safe to predict that the treatment space will significantly impact the GIST market during the forecast period. Moreover, the anticipated introduction of emerging therapies with improved efficacy and a further improvement in the diagnosis rate is expected to drive the growth of the GIST market in the 7MM.
However, several factors may impede the growth of the GIST market. One of the key challenges is the development of resistance to current therapies, particularly tyrosine kinase inhibitors, which limits long-term treatment efficacy. Additionally, the rarity of GIST poses challenges for large-scale clinical trials, slowing down the pace of drug development and approval. High treatment costs and limited access to advanced therapies in low- and middle-income countries further restrict market penetration.
Moreover, GIST treatment poses a significant economic burden and disrupts patients’ overall well-being and QOL. Furthermore, the Gastrointestinal Stromal Tumor market growth may be offset by failures and discontinuation of emerging therapies, unaffordable pricing, market access and reimbursement issues, and a shortage of healthcare specialists. In addition, the undiagnosed, unreported cases and the unawareness about the disease may also impact the GIST market growth.
| Gastrointestinal Stromal Tumor Report Metrics | Details |
| Study Period | 2020–2034 |
| Gastrointestinal Stromal Tumor Report Coverage | 7MM [The United States, the EU-4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan] |
| Gastrointestinal Stromal Tumor Market Size in 2023 | USD 450 Million |
| Key Gastrointestinal Stromal Tumor Companies | Cogent Biosciences, IDRx, Deciphera Pharmaceuticals, Novartis, Bayer, Pfizer, Blueprint Medicines Corporation, Loxo Oncology, and others |
| Key Gastrointestinal Stromal Tumor Therapies | Bezuclastinib (CGT9486), IDRX-42, Ripretinib, and others |
Scope of the Gastrointestinal Stromal Tumor Market Report
- Gastrointestinal Stromal Tumor Therapeutic Assessment: Gastrointestinal Stromal Tumor current marketed and emerging therapies
- Gastrointestinal Stromal Tumor Market Dynamics: Conjoint Analysis of Emerging Gastrointestinal Stromal Tumor Drugs
- Competitive Intelligence Analysis: SWOT analysis and Market entry strategies
- Unmet Needs, KOL’s views, Analyst’s views, Gastrointestinal Stromal Tumor Market Access and Reimbursement
Discover more about Gastrointestinal Stromal Tumor drugs in development @ Gastrointestinal Stromal Tumor Clinical Trials
Table of Contents
| 1. | Gastrointestinal Stromal Tumor Market Key Insights |
| 2. | Gastrointestinal Stromal Tumor Market Report Introduction |
| 3. | Gastrointestinal Stromal Tumor Market Overview at a Glance |
| 4. | Gastrointestinal Stromal Tumor Market Executive Summary |
| 5. | Disease Background and Overview |
| 6. | Gastrointestinal Stromal Tumor Treatment and Management |
| 7. | Gastrointestinal Stromal Tumor Epidemiology and Patient Population |
| 8. | Patient Journey |
| 9. | Gastrointestinal Stromal Tumor Marketed Drugs |
| 10. | Gastrointestinal Stromal Tumor Emerging Drugs |
| 11. | Seven Major Gastrointestinal Stromal Tumor Market Analysis |
| 12. | Gastrointestinal Stromal Tumor Market Outlook |
| 13. | Potential of Current and Emerging Therapies |
| 14. | KOL Views |
| 15. | Unmet Needs |
| 16. | SWOT Analysis |
| 17. | Appendix |
| 18. | DelveInsight Capabilities |
| 19. | Disclaimer |
| 20. | About DelveInsight |
Related Reports
Gastrointestinal Stromal Tumor Pipeline
Gastrointestinal Stromal Tumor Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key GIST companies, including Jiangsu Hengrui Medicine, Daiichi Sankyo Company, Cogent Biosciences, Advenchen Laboratories, Chia Tai Tianqing Pharmaceutical Group, AB Science, Immunicum AB, Novartis, Bristol-Myers Squibb, Hanmi Pharmaceutical Company Limited, GlaxoSmithKline, Ascentage Pharma, Takeda, Array BioPharma, Plexxikon, Arog Pharmaceuticals, Xencor, Inc., DNAtrix, Inc., Onyx Pharmaceuticals, Exelixis, Allarity Therapeutics, Theseus Pharmaceuticals, IDRx, Inc., Allarity Therapeutics, among others.
Gastrointestinal Stromal Tumor Epidemiology Forecast
Gastrointestinal Stromal Tumor Epidemiology Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted GIST epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
NTRK Market Size, Target Population, Competitive Landscape & Market Forecast – 2034 report deliver an in-depth understanding, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key NTRK companies including iOMEDICO AG, Bayer, Pyramid Biosciences, Hoffmann-La Roche, Beijing InnoCare Pharma Tech Co., Ltd., Jiangsu Simcere Pharmaceutical Co., Ltd., Plexxikon, AnHeart Therapeutics Inc., Fochon Pharmaceuticals Ltd., among others.
TRK Fusion Cancer Market Insight, Epidemiology And Market Forecast – 2034 report deliver an in-depth understanding, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key TRK fusion cancer companies including Bayer, Pyramid Biosciences, Exelixis, Beijing InnoCare Pharma Tech Co., Ltd, Fochon Pharmaceuticals, Ltd, Daiichi Sankyo Co., Ltd, among others.
TRK Fusion Cancer Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key TRK fusion cancer companies, including Bayer, Pyramid Biosciences, Exelixis, Beijing InnoCare Pharma Tech Co., Ltd, Fochon Pharmaceuticals, Ltd, Daiichi Sankyo Co., Ltd, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter

Contact Us
Shruti Thakur
info@delveinsight.com
+14699457679
www.delveinsight.com
Distribution channels: Healthcare & Pharmaceuticals Industry, Media, Advertising & PR, Science ...
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release

